
Clinical evidence
EBI’s lead product, EA-230, helps patients recover faster from major surgeries and reduces hospital costs.

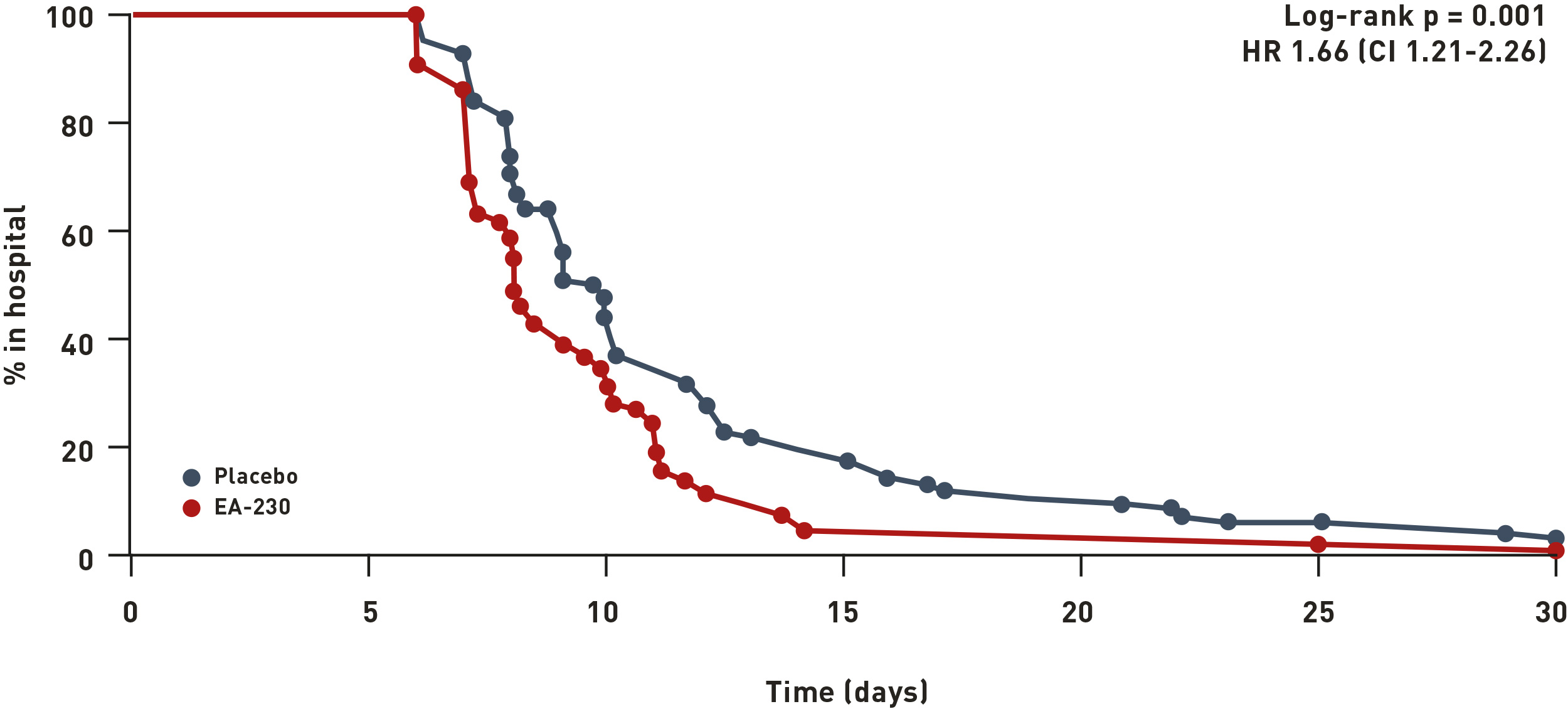
Phase II
To evaluate its use in major surgery, an intravenous solution of EA-230 was given to patients during cardiac surgery comprising Coronary Artery Bypass Grafting (CABG) and valve replacement. Length-of-stay in the hospital of EA-230-treated patients was significantly reduced compared to LoS of placebo-treated patients (see image above). Placebo patients required 2.5 times longer duration of treatment dependent postoperative complications (p=0.0026) and demonstrated 25% longer length-of-stay (p=0.001) than EA-230 patients.
Yearly, ~400.000 CABG surgeries are performed in the USA. Under 2024 USA-pricing, the Phase II study results show that treatment with EA-230 may provide savings of $33,420 on hospital stay per patient. To allow for maximum utilization of the potential upside that resides in cost savings of > $4,5 billion/year in the field of CABG in the USA, already alone.
For further information about our Phase II results please see pdf.
Phase III
With the data obtained in previous studies, the risks of taking EA-230 to a Phase III program are considered limited. The design for this program has been discussed with the FDA and focuses on confirming the positive results from the Phase IIb study.
The Phase III clinical development program for EA-230 consists of international, multicentre, randomized, double-blind, placebo-controlled superiority clinical Phase III studies in major surgery patients to confirm safety and efficacy of EA-230. The Phase III study will include cardiac surgery patients, including CABG and valve replacement surgery, and has been designed with the FDA to confirm the efficacy of EA-230 on in hospital length-of-stay as primary endpoint, with manifestations of post operative complications as key secondary endpoints.
With a successful completion of the Phase III trial, EBI aims at applying for market authorization.

